For Immediate Release
Company name: DAIICHI SANKYO COMPANY, LIMITED
Representative: Joji Nakayama, Representative Director, President and CEO
(Code no.: 4568, First Section, Tokyo Stock Exchange)
Please address inquiries to Noriaki Ishida, Executive Officer,
Vice President, Corporate Communications Department
Telephone: +81-3-6225-1126
http://www.daiichisankyo.com
Daiichi Sankyo Launches New Generic Drugs through Its Daiichi Sankyo Espha Subsidiary
Tokyo, Japan (December 8, 2016) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that on December 9, 2016, its generics subsidiary, Daiichi Sankyo Espha Co., Ltd (hereinafter, Daiichi Sankyo Espha), will launch five new generic drugs with three new active ingredients, which have been notified through today’s official gazette.
Overview of Newly Released Products
1. Product Names/Therapeutic Categories
|
Product name
|
Therapeutic category
|
Original brand name
|
|
Montelukast Tablets 5mg “DSEP”
|
Leukotriene receptor antagonist/
Bronchial asthma/allergic rhinitis treatment
|
Singulair® Tablets 5mg, 10mg
KIPRES® Tablets 5mg, 10mg
|
|
Montelukast Tablets 10mg “DSEP”
|
|
Pramipexole Hydrochloride LA Tablets 0.375mgMI “DSEP”
|
Dopaminergic Parkinson's disease treatment
Sustained-release preparation
|
Mirapex®-LA Tablets 0.375mg, 1.5mg
|
|
Pramipexole Hydrochloride LA Tablets 1.5mgMI “DSEP”
|
|
Bosentan Tablets 62.5mg “DSEP”
|
Endothelin receptor antagonist
|
Tracleer® Tablets 62.5mg
|
2. Product Attributes
1) Innovative design of PTP Sheets
Ongoing innovations in formulation and labeling reduce the burden on pharmacists when confirming and preventing drug error, and prevent medical error, such as patients taking the wrong medicine or confusing one medicine with another.
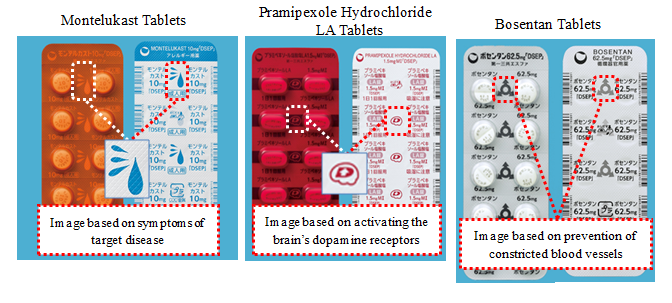
・Easily recognizable original symbols PTP sheets feature original symbols based on formulation and disease characteristics.
・GS1 code on each tablet
GS1 dispenser packaging unit code is displayed on the back of the PTP sheet to reduce the burden on pharmacists in preventing drug error.
・Pitch control (fixed position printing)
Makes it easier to identify product name, active ingredient or DSEP display.
・Other innovations
Adopted material that allows content confirmation along with coloring for light shielding for Montelukast Tablets and Pramipexole Hydrochloride LA Tablets.
2) Innovative design of tablets
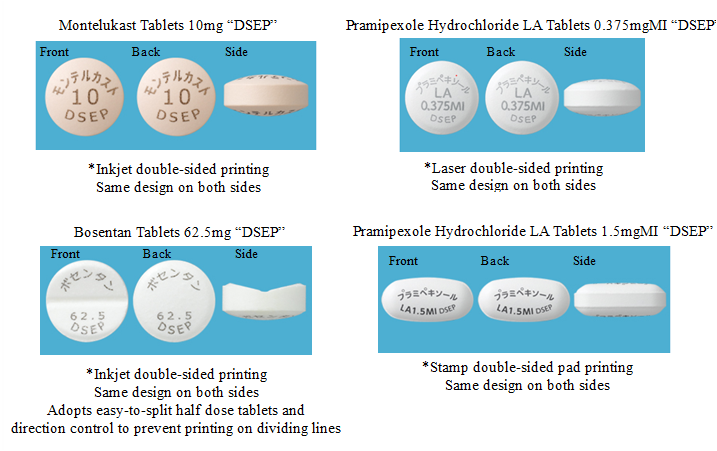
・Double-sided printing
Product name, active ingredient, etc. are printed on both sides using inkjet, laser or stamp pad printing, according to the characteristics of each method, making print easy to read.
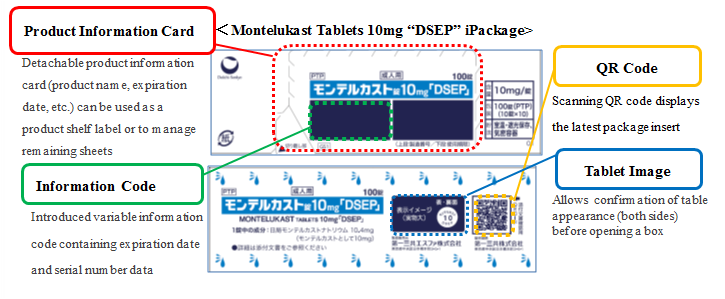
3) Innovative Box Design
Introducing currently designed boxes (iPackage) for some new products enabling to check the various information when dispensing.
About Daiichi Sankyo Espha
Daiichi Sankyo Espha provides innovative high value-added generic drugs with formulation and devisal labeling as well as authorized generics which are manufactured from the same substances and additives that are manufactured in the same manufacturing methods in the same plant as original drugs based on the spirit of the Daiichi Sankyo Group corporate mission* of supplying pharmaceuticals to address diverse medical needs. Daiichi Sankyo Espha strives to provide pharmaceuticals that provide peace of mind for users by fulfilling the most important pharmaceutical criteria of quality, information, and stable supply while delivering the economic benefits of generics.
*Daiichi Sankyo Group corporate mission: To contribute to the enrichment of quality of life around the world through the creation of innovative pharmaceuticals, and through the provision of pharmaceuticals addressing diverse medical needs.
Daiichi Sankyo Espha Company Overview
Company name: Daiichi Sankyo Espha Co., Ltd.
Established: April 1, 2010
Business: Manufacture and sale of pharmaceuticals
Capital: 450 million yen
Representative: President Hiroto Yoshiwaka
Headquarters: 3-5-1, Nihonbashi, Honcho, Chuo-ku, Tokyo